
THE FROGGYMOUTH IS MADE OF THERMOPLASTIC ELASTOMER
(TPE) AND CONTAINS NO LATEX OR PHTHALATE.
IT IS PRODUCED ACCORDING TO ISO9001 AND ISO13485 STANDARDS
WHICH ENSURE IDENTICAL PRODUCTION QUALITY
OF EACH OF OUR DEVICES.
IT IS CLASSIFIED AS A CLASS I MEDICAL DEVICE,
NOT STERILIZABLE ACCORDING TO THE RULES
OF EUROPEAN CERTIFICATION (CE).
FDA, HEALTH CANADA, ETC. CERTIFICATIONS ALLOW
INTERNATIONAL MARKETING OF THE DEVICE.






DESIGN
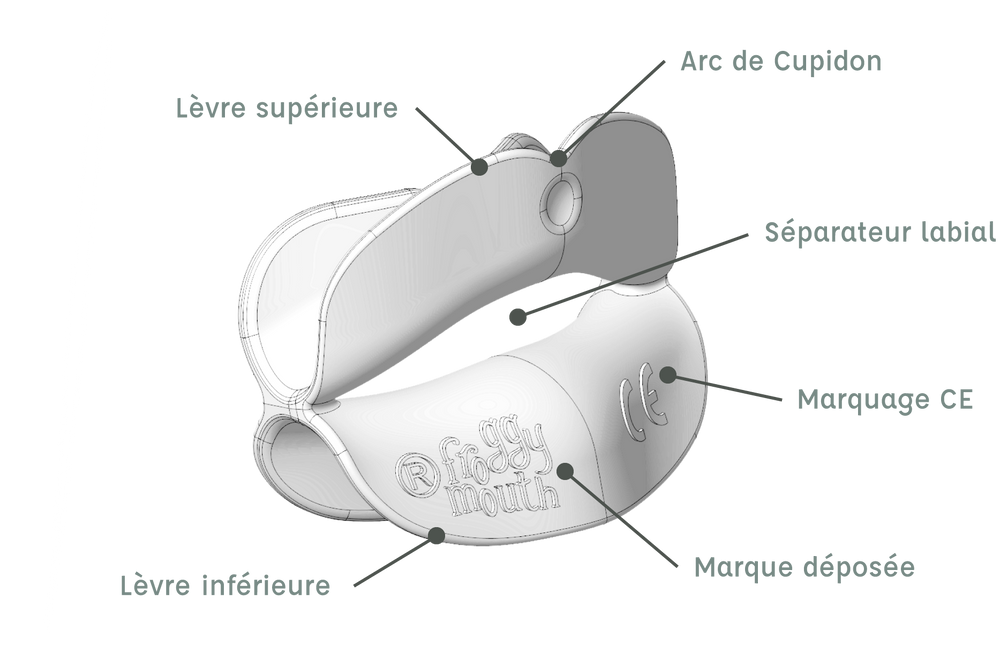
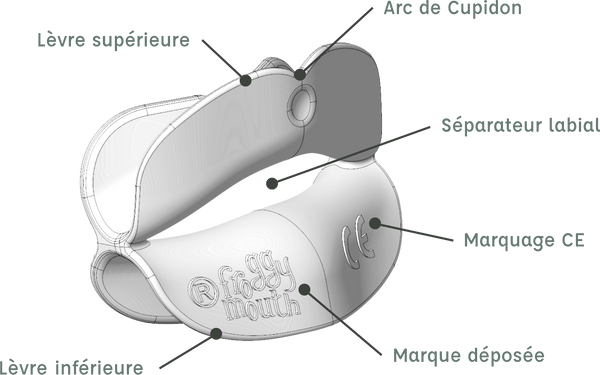
DESIGN COMPUTER-AIDED DESIGN IS THE FIRST PART OF THE PRODUCTION PROCESS. IT ALLOWS TRANSITIONING FROM A TWO-DIMENSIONAL SKETCH STAGE TO A THREE-DIMENSIONAL VISUAL DESIGN.

PRODUCTION
THE THERMOPLASTIC ELASTOMER (T.P.E) IS HEATED AND THEN INJECTED INTO THE MOLDS. EVERYTHING IS MONITORED TO ENSURE STRICT PRODUCTION CONDITIONS ARE MET.

PACKAGING
EACH FROGGYMOUTH IS THEN METICULOUSLY PACKAGED BY THE WORKERS OF THE WISE (WORK INTEGRATION SOCIAL ENTERPRISE) OF NANTES.



